Severe congenital neutropenia (SCN) is characterized by severe neutropenia and recurrent episodes of critical infections. X-linked neutropenia (XLN) is a very rare type of SCN caused by a gain-of-function mutation in the Wiscott-Aldrich syndrome gene (WAS), the product of which (WASp) is expressed only in blood cells, and especially to higher extent during neutrophil maturation. Only four types of WASp mutations in its GBD domain (L270P, S272P, I290T, and I294T) have been described so far, and we reported the first Asian case with WASp-I290T.These mutations are supposed to destroy the autoinhibiting conformation of WASp and aberrantly activates WASp. Two mouse models harboring WASp-L272P or I296T, mouse homologs of XLN WASp, have been developed, but neither could recapitulate neutropenia. Herein, we established a novel knock-in mouse carrying WASp-I292T, a mouse homolog of XLN WASp-I290T, and we combined investigations of its peripheral blood and bone marrow cells with analysis of primary samples and iPS cells derived from a WASp-I290T patient.
A proband of XLN had 1.3 x 103/mL WBC with a differential count of 8% neutrophils, 5.3% monocytes, 2.3% eosinophils, 3% basophils, and 80.4% lymphocytes. BM revealed marked myeloid hypoplasia with a slight dysplastic change and maturation arrest at the myelocyte stage, and colony forming ability of BM CD34+cells in methylcellulose containing SCF, IL3, and G-CSF demonstrated profound defects in G- and GM-colonies. The patient-derived iPS cells were developed and were induced to differentiate into HSCs by a coculture over an OP9 monolayer, supplemented with optimal cytokine cocktails. Resulting frequency of CD45+CD34+cells was rather comparable to that from cord blood (CB)-derived iPS cells. However, XLN-iPS-derived CD45+CD34+cells produced 5-fold smaller number of myeloid colonies than CB-iPS-derived thosecells in the same methylcellulose culture.
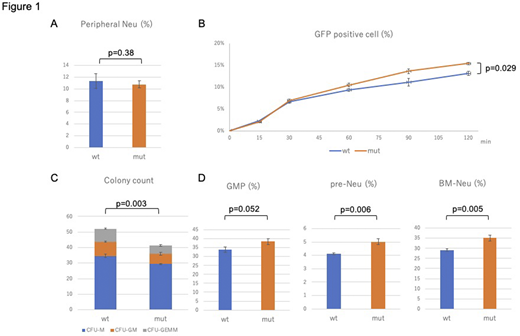
To further gain insight into the underlying abnormalities induced by WASp-I290T, we established a novel knock-in mouse model harboring WASp-I292T, which was obtained from CRISPR/Cas9-engineered mouse ES cells. Unlike XLN patients, but similar to two earlier mouse studies, the WASp-I292T mice had normal count of neutrophils, monocytes, B cells, CD4+ T cells, CD8+ T cells, and NK cells (Figure 1A). Administration of G-CSF into the WASp-I292T mice increased neutrophils to the comparable level to Wt mice. Similarly, lipopolysaccharide increased neutrophils and monocytes comparable to Wtmice. However, WASp-I292T neutrophils possessed a stronger ability of phagocytosis than Wt, when determined by the uptake of fluorescently pre-labeled E. coli(p=0.029) (Figure 1B).
Colony forming ability of the bone marrow c-kit+, sca-1+, lin-(KSL) cells in methylcellulose containing EPO, IL-3, IL-6, and SCF was significantly impaired in mut mice (p=0.003) (Figure 1C). In the bone marrow, however, the fraction of granulocyte/monocyte progenitors (GMPs; c-kit+, sca-1-, CD34+, CD16/32-) and pre-neutrophils (c-kit+, Gr-1dim, CXCR2-, CXCR4+) were marginally increased in the mut mice (p=0.052, and p=0.006, respectively), and megakaryocyte/erythroid progenitors (MEP; c-kit+, sca-1-, CD34-, CD16/32-) was slightly decreased in the mut mice (p=0.051) (Figure 1D). These results suggested that granulopoietic potential was basically maintained in the WASp-I292T mice, but the process at the end of neutrophil production or the egression from the bone marrow were impaired. We previously reported that NB4WASp-I290T cells produces significantly fewer neutrophils following ATRA-induced differentiation. In addition, analysis of myeloid-related genes revealed a premature upregulation of C/EBP-epsilon in NB4WASp-I290T cells. Because WASp contains a nuclear localization signal-like motif in the basic domain, and confocal laser microscopy suggested that WASp-I290T preferentially localizes in the nucleus rather than cytoplasm, it is likely that WASp-I290T alters myeloid-related genes by direct or indirect interactions with transcription factors in the nucleus; although detailed mechanisms still remain to be elucidated. As compared with profound defects in XLN-patients, defects in WASp-I290T mice were quite modest, suggesting some compensatory mechanisms in the murine hematopoiesis. Further studies of transcription factors and protein-protein interactions would provide a more precise explanation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal